Answer:
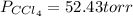
Step-by-step explanation:
Hello there!
In this case, sine the solution of this problem require the application of the Raoult's law, assuming heptane is a nonvolatile solute, so we can write:
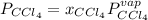
Thus, we first calculate the mole fraction of chloroform, by using the given masses and molar masses as shown below:
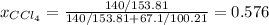
Therefore, the partial pressure of chloroform turns out to be:
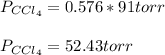
Regards!