Answer:
a): The name of the product nuclide is lead-214
b): The symbol of the product nuclide is Pb-218
Step-by-step explanation:
There are three types of decay processes:
- Alpha decay
- Beta decay
- Gamma decay
Alpha decay is the decay process that happens when a heavy nucleus decays into a light nucleus with the release of an alpha particle. This alpha particle carries a charge of +2 units and has a mass of 4 units. It is also known as the helium nucleus. The general equation for this decay process is:
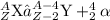
The nuclear equation for the alpha decay of Po-218 follows:
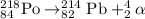
Hence, the name of the product nuclide is lead-214 and the symbol is Pb-218.