Answer:
The half-life of the substance is of 1600 years.
Explanation:
Amount of the substance:
The amount of the substance after t years is given by the following equation:
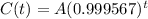
In which A is the initial amount.
Find the half-life:
This is t for which
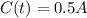 , that is, the amount is half the initial amount. So
, that is, the amount is half the initial amount. So
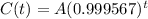
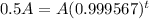
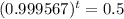
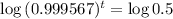
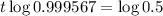
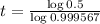
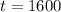
The half-life of the substance is of 1600 years.