Answer: The mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid according to the given balanced equation is 3.03 g.
Step-by-step explanation:
The given balanced reaction equation is as follows.
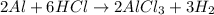
Here, the mole ration of Al and hydrogen produced is 2 : 3
As mass of aluminum is given as 26.98 g. So, moles of aluminum (molar mass = 26.98 g/mol) is as follows.
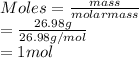
So, when 1 mole of Al reacted then 1.5 moles of hydrogen is produced as per the given mole ratio.
Therefore, mass of hydrogen formed is calculated as follows.
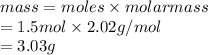
Thus, we can conclude that the mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid according to the given balanced equation is 3.03 g.