Answer:
There are
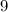 electrons in this atom.
electrons in this atom.
Step-by-step explanation:
Electron configuration of this atom:
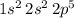 .
.
The electron orbitals of an atom are denoted as
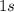 ,
,
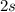 ,
,
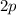 ,
,
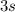 ,
,
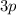 , etc. At any given time, an electron in this atom is located in exactly one orbital.
, etc. At any given time, an electron in this atom is located in exactly one orbital.
The electron configuration of an atom gives the number of electrons in each orbitals of this atom.
For example, in this atom, the superscript "
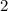 " on the right of "
" on the right of "
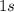 " means that there are two electrons in the
" means that there are two electrons in the
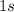 orbital of this atom. Hence,
orbital of this atom. Hence,
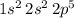 would translate to:
would translate to:
- The
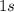 orbital of this atom contains
orbital of this atom contains
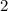 electrons.
electrons. - The
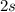 orbital of this atom contains
orbital of this atom contains
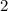 electrons.
electrons. - The
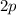 orbitals of this atom contain
orbitals of this atom contain
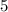 electrons.
electrons.
Hence, there would be
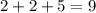 electrons in total in this atom.
electrons in total in this atom.