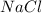Answer: The chemical symbol of the compound formed is
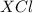 or
or
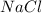
Step-by-step explanation:
An ionic compound is formed when the complete transfer of electrons takes place from one element (usually metals) to another element (usually non-metals).
The element losing electron (metal) forms a positive ion known as a cation while the element gaining electron (non-metal) forms a negative ion known as an anion.
Element X has the atomic number 11 which is sodium. The electronic configuration of it is
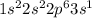
This element will lose 1 electron to form
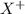 ion of
ion of
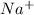
Chlorine has the atomic number 17. The electronic configuration of it is
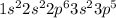
This element will gain 1 electron to form
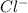 ion
ion
When these two ions combine, they lead to the formation of an ionic compound having a chemical formula of
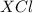 or
or
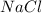
Hence, the chemical symbol of the compound formed is
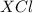 or
or