Answer: The mass of water required is 18.52 g
Step-by-step explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass.
The equation used is:
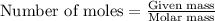 ......(1)
......(1)
Given mass of magnesium hydroxide = 30.0 g
Molar mass of magnesium hydroxide = 58.32 g/mol
Plugging values in equation 1:
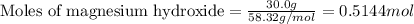
The given chemical equation follows:
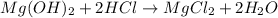
By the stoichiometry of the reaction:
If 1 mole of magnesium hydroxide produces 2 moles of water
So, 0.5144 moles of magnesium hydroxide will react with =
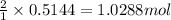 of water
of water
Molar mass of water = 18 g/mol
Plugging values in equation 1:
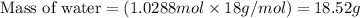
Hence, the mass of water required is 18.52 g