Answer:
The volume of the solution is 3 L.
Step-by-step explanation:
Molarity is a measure of the concentration of that substance that indicates the number of moles per unit volume. In other words, molarity is the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of the solute by the volume of the solution.
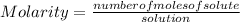
Molarity is expressed in units
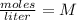
In this case:
- Molarity= 0.25 M
- number of moles of solute= 0.75 moles
- volume= ?
Replacing in the definition of molarity:
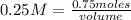
volume*0.25 M= 0.75 moles
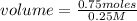
volume= 3 L
The volume of the solution is 3 L.