Answer: The temperature of the unknown gas is 191.4 K.
Step-by-step explanation:
To calculate the temperature of the gas, we use ideal gas equation which is given as:
 .......(1)
.......(1)
Where
P = pressure of the gas = 5.5 atm
V = volume of gas = 12.0 L
n = number of moles = 4.2 moles
R = Gas constant = 0.0821 L.atm/mol.K
T = temperature of the gas = ?
Putting values in equation 1:
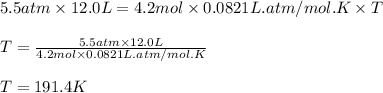
Hence, the temperature of the unknown gas is 191.4 K.