Answer:
0.159 M (3 s.f.)
Step-by-step explanation:
This is an acid- base reaction, which is also known as a neutralisation reaction.
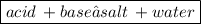
1) Write out the balanced chemical equation.
NaOH (aq) +HCl (aq) → NaCl (aq) +H₂O(l)
The equation is balanced as on each side of the equation, there is 1 Na atom, 1 O atom, 2 H atoms and 1 Cl atom.
2) Find the number of moles of NaOH.
The symbol 'M' denotes Molarity, which is the number of moles per litre.
From the given concentration of NaOH,
1 L NaOH ----- 0.450 mol NaOH
Convert 83.0mL to litres:
Volume of NaOH
= 83 ÷1000
= 0.083 L
Moles of NaOH
= 0.083 ×0.450
= 0.03735 mol
3) Find the number of moles of HCl.
From the balanced equation, 1 mole of HCl is needed to neutralize 1 mole of NaOH.
Thus, amount of HCl
= 0.03735 mol
4) Calculate the concentration of HCl
Concentration= mol/ volume
Converting the volume from mL to L,
235 mL= 0.235 L
Concentration of HCl
= 0.03735 ÷0.235
= 0.15894 mol/L (5 s.f.)
= 0.159 M (3 s.f.)