Answer:
Given theoretical yield of NH3 is 945g.
The actual yield is 598g.
What is the %yield?
Step-by-step explanation:
%yield of a chemical reaction can be calculated by using the formula:

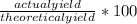
Substitute the given values in this formula to get the %yield.

Hence, the %yield for the formation of ammonia is ---- 63.3.