Answer:
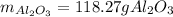
Step-by-step explanation:
Hello there!
In this case, if we consider the following chemical reaction, whereby Al2O3 is produced from Al and FeO:
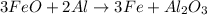
Thus, since there is 3:1 mole ratio of FeO to Al2O3, it turns out feasible for us to use their molar masses, 71.844 g/mol and 101.96 g/mol respectively, to obtain the grams of the latter as follows:
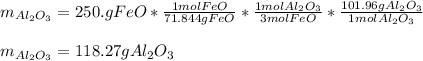
Regards!