Answer:
A compressed gas cylinder is filled with 5270 g of argon gas.
The pressure inside the cylinder is 2050 psi at a temperature of 18C.
The valve to the cylinder is opened and gas escapes until the pressure inside the cylinder is 650. psi and the temperature are 26 C.
How many grams of argon remains in the cylinder?
Step-by-step explanation:
First, calculate the volume of argon gas that is present in the gas cylinder by using the ideal gas equation:
Mass of Ar gas is --- 5270g.
The number of moles of Ar gas:
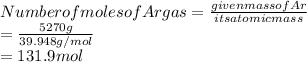
Temperature T=(18+273)K=291K
Pressure P=2050psi
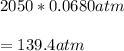
Volume V=?

Using this volume V=22.6L
Pressure=650psi=44.2atm
Temperature T= (26+273)K=299K
calculate number of moles "n" value:
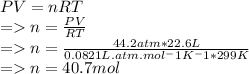
Mass of 40.7mol of Ar gas:
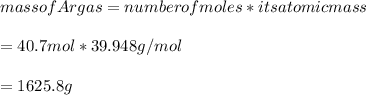
Answer:
The mass of Ar gas becomes 1625.8g.