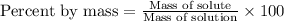Answer: The equation for percent by mass is
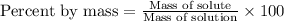
Step-by-step explanation:
A solution consists of solute and solvent.
A solute is defined as a component that is present in a smaller proportion.
A solvent is defined as a component that is present in a larger proportion.
Concentration is defined as the amount of solute present in the given amount of solution or solvent.
The percent by mass is defined as the concentration of a solute where the ratio of the mass of solute is taken with respect to the mass of solvent multiplied by 100.
The equation used is: