Answer:
1.115 g
Step-by-step explanation:
Applying,
R = R'
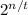 ................... Equation 1
................... Equation 1
Where R = original sample of radon-222, R' = sample of radon-222 left after decay, n = Total time, t = half-life.
make R' the subject of the equation
R' = R/(
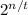 )............... Equation 2
)............... Equation 2
From the question,
Given: R = 73.9 g, n = 23 days, t = 3.8 days.
Substitute these values into equation 2
R' = 73.9/(
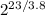 )
)
R' = 73.9/
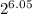
R' = 1.115 g