Answer:
For 2: The % yield of the product is 92.34 %
For 3: 12.208 L of carbon dioxide will be formed.
Step-by-step explanation:
The percent yield of a reaction is calculated by using an equation:
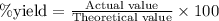 ......(1)
......(1)
Given values:
Actual value of the product = 78.4 g
Theoretical value of the product = 84.9 g
Plugging values in equation 1:
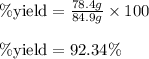
Hence, the % yield of the product is 92.34 %
The number of moles is defined as the ratio of the mass of a substance to its molar mass.
The equation used is:
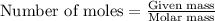 ......(2)
......(2)
Given mass of carbon dioxide = 24 g
Molar mass of carbon dioxide = 44 g/mol
Plugging values in equation 1:
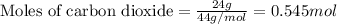
At STP conditions:
1 mole of a gas occupies 22.4 L of volume
So, 0.545 moles of carbon dioxide will occupy =
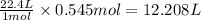 of volume
of volume
Hence, 12.208 L of carbon dioxide will be formed.