The complete question is: Fill the blanks with appropriate coefficient number for the reaction equation- __AgNO3 + __Cu → __Ag + __Cu(NO3)2
Answer: The complete reaction equation is
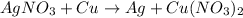
Step-by-step explanation:
A chemical equation which contains same number of atoms on both reactant and product side is called a balanced chemical equation.
For example,
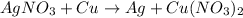
Here, number of atoms present on reactant side is as follows.
- Ag = 1
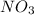 = 1
= 1- Cu = 1
Number of atoms present on product side is as follows.
- Ag = 1
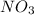 = 2
= 2- Cu = 1
To balance this equation, multiply
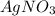 by 2 on reactant side and multiply Ag by 2 on product side. Hence, the equation can be re-written as follows.
by 2 on reactant side and multiply Ag by 2 on product side. Hence, the equation can be re-written as follows.
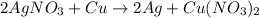
Now, the number of atoms present on reactant side are as follows.
- Ag = 2
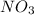 = 2
= 2- Cu = 1
Number of atoms present on product side are as follows.
- Ag = 2
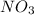 = 2
= 2- Cu = 1
Thus, we can conclude that the complete reaction equation is
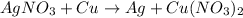 .
.