Answer:
Step-by-step explanation:
First off, let's call the -15 degree water solution1, and the 32 degree water solution2. The formula for this is
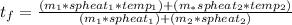
where spheat is specific heat of water that I found to be 4182 J/(kgC). This means that we need our masses in kg and they are currently in g.
100 g = .100 kg and
220 g = .220 kg. Now we're ready to plug everything in:
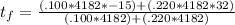 and
and
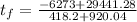 and
and
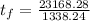 so
so
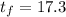 °C
°C