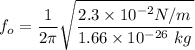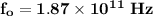Answer:
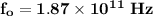
Step-by-step explanation:
From the given information:
The elastic potential energy can be calculated by using the formula:
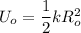
Making K the subject;
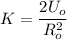
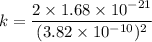
k = 2.3 × 10⁻² N/m
Now; the frequency of the small oscillation can be determined by using the formula:
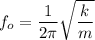
where;
m = mass of each atom = 1.66 × 10⁻²⁶ kg