Answer:
23.2 kJ of energy are released by the reaction.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary for us to calculate the moles of both tin and nitrogen and the produced moles of Sn3N4 product by each reactant as shown below:
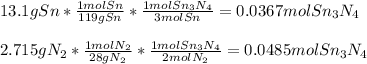
Thus, since 13.1 grams of tin produce the fewest moles of Sn3N4 product, we infer tin is the limiting reactant, and the correct produced energy, due to this reaction is:
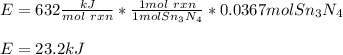
Regards!