Answer:
NaOH is the limiting reactant.
Step-by-step explanation:
Hello there!
In this case, since the reaction taking place between sodium hydroxide and chlorine has is:
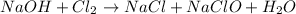
Which must be balanced according to the law of conservation of mass:
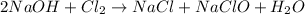
Whereas there is a 2:1 mole ratio of NaOH to Cl2, which means that the moles of the former that are consumed by 0.85 moles of the latter are:
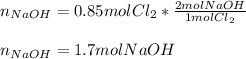
Therefore, since we just have 1.23 moles out of 1.70 moles of NaOH, we infer this is the limiting reactant.
Regards!