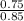Answer:
pH = 3.68
Step-by-step explanation:
We can solve this problem by using Henderson-Hasselbach's equation:
- pH = pKa + log
![([Formate])/([Formic Acid])](https://img.qammunity.org/2022/formulas/chemistry/college/teixo5boajc5lr9bhykhqt58rpa0qwtx59.png)
- pKa = -log(1.8x10⁻⁴) = 3.74
Assuming we have 1 L of the buffer solution then the molar concentrations of formate and formic acid would be:
- [Formate] = 0.75 mol / 1 L = 0.75 M
- [Formic Acid] = 0.85 mol / 1 L = 0.85 M
We now have all required data to calculate the pH:
- pH = 3.74 + log