Answer:
Y = 92.5 %
Step-by-step explanation:
Hello there!
In this case, since the reaction between lead (II) nitrate and potassium bromide is:
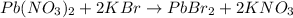
Exhibits a 1:2 mole ratio of the former to the later, we can calculate the moles of lead (II) bromide product to figure out the limiting reactant:
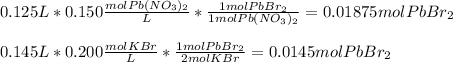
Thus, the limiting reactant is the KBr as it yields the fewest moles of PbBr2 product. Afterwards, we calculate the mass of product by using its molar mass:
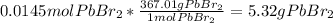
And the resulting percent yield:
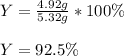
Regards!