Answer:
d . The volume of the solvent used was less than 5 liters.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the volume of the stock (initial) solution by using the following equation:
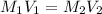
Thus, we solve for, V1, which stands for the aforementioned volume of stock solution:
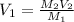
Then, we plug in to obtain:
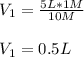
Now, since the final volume was 5 L, we can infer that the volume of solvent is 4.5 L and that of the stock solution 0.5 L for a total of 5 L of diluted solution; therefore, the correct reasoning is d . The volume of the solvent used was less than 5 liters.
Regards!