Answer: The oxidation state of Al in
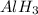 is +3.
is +3.
Step-by-step explanation:
The number attained by an atom or substance due to loss or gain of electrons is called oxidation state.
For example, in
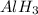 it is known that the oxidation state of hydrogen is -1 as aluminum is a cation so it cannot have an oxidation state as 'minus'.
it is known that the oxidation state of hydrogen is -1 as aluminum is a cation so it cannot have an oxidation state as 'minus'.
Whereas hydrogen has the property to act both as a cation and an anion.
Let us assume the oxidation state of Al is x in the compound
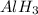 .
.
Hence, oxidation state of Al is calculated as follows.
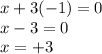
This means that the oxidation state of Al in
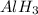 is +3.
is +3.
Thus, we can conclude that oxidation state of Al in
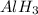 is +3.
is +3.