Answer:
48 moles of water
Step-by-step explanation:
2C2H6 + 7O2 --> 4CO2 + 6H2O
We want to find the number of moles of water produced if 16 moles of ethane (C2H6) are burned.
We can use dimensional analysis to find the answer.
Needed conversions: We need the mole ratio of ethane to water in the chemical equation which would be 2 to 6. So for every 2 moles of ethane that are burned 6 moles of water is produced.
Using this ratio we can create a table:
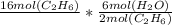
the mol (C2H6) cancel out and we get
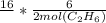
we then multiply
16 * 6/2molC2H6 = 48molH2O
So if 16 moles of ethane is burned 48 moles of water is produced