Answer: The given decay sequence is
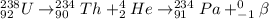 .
.
Step-by-step explanation:
An alpha-particle is a helium atom. Hence, when an alpha decay occurs in
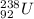 then the reaction equation is as follows.
then the reaction equation is as follows.
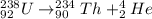
Now, in sequence the equation for beta decay is as follows.
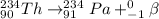
Hence, the sequence will be as follows.
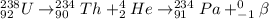
Thus, we can conclude that the given decay sequence is
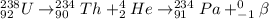 .
.