Answer:
150
Step-by-step explanation:
- C₄H₂OH + 6O2 → 4CO2 + 5H₂O
We can find the equivalent number of O₂ molecules for 100 molecules of CO₂ using a conversion factor containing the stoichiometric coefficients of the balanced reaction, as follows:
- 100 molecules CO₂ *
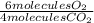 = 150 molecules O₂
= 150 molecules O₂
150 molecules of O₂ would produce 100 molecules of CO₂.