Answer: A volume of 10.66 mL of 1.5 M H is required to exactly neutralize 20. milliliters of a 0.8 M KOH solution.
Step-by-step explanation:
Given:
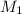 = 1.5 M,
= 1.5 M,
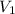 = ?
= ?
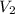 = 20 mL,
= 20 mL,
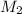 = 0.8 M
= 0.8 M
Formula used to calculate the volume is as follows.
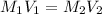
Substitute the values into above formula as follows.
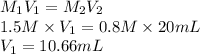
Thus, we can conclude that a volume of 10.66 mL of 1.5 M H is required to exactly neutralize 20. milliliters of a 0.8 M KOH solution.