Answer:
Boyle's law, Charle's law, Guy Lussac's law and Avogadro's law
Step-by-step explanation:
All the gases behaves similarly when the environment conditions are normal. But when the physical condition changes like when the pressure, volume or temperature changes, the gas behaves differently and shows a deviation.
The number of gas laws are :
Boyle's Law
Boyle's law states that when the temperature remaining constant, the pressure of the gas varies inversely to the volume of the gas.
i.e.
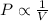
Charle' law
Charle's law states that when pressure is constant, the temperature of a gas is directly proportional to the volume.
i.e. ,
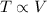
Gay Lussac's law
Gay - Lussa law states the volume and the mass of the pressure of the gas is directly proportional to the temperature of the gas.
i.e. P.T = constant
Avogadro's law
It states that under the conditions of same pressure as well as temperatures, the gases having equal volumes will have same numbers of molecules.
i.e.
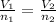 = constant
= constant