Answer:
Aluminum
Step-by-step explanation:
Given
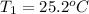
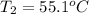
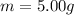
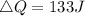
See attachment for chart
Required
Identify the unknown substance
To do this, we simply calculate the specific heat capacity from the given parameters using:
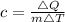
This gives:
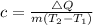
So, we have:
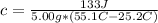
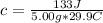
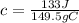
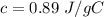
From the attached chart, we have:
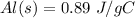 --- The specific heat capacity of Aluminum
--- The specific heat capacity of Aluminum
Hence, the unknown substance is Aluminum