Answer:
.265 mol
Step-by-step explanation:
We want to find the number of moles in 15.9 grams of SiO2
First we need to find the molar mass of SiO2
Looking at a periodic table 1 atom of Si has a mass of 28.086g
And 1 atom of oxygen has a mass of 15.999g
So the molar mass of SiO2 would be 28.086(1) + 15.999(2) = 60.084g/mol
Now we can use dimensional analysis to find the number of moles in 15.9 grams of SiO2
Setting up the table we get
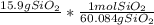
the g SiO2 cancels out and we get
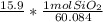
we then evaluate 15.9 * 1/60.084 to get .265 mol
So there are .265 moles in 15.9 grams of SiO2