Answer:
The number of electrons transferred from one ball to the other is 2.06 x 10¹² electrons
Step-by-step explanation:
Given;
magnitude of the attractive force, F = 17 mN = 0.017 N
distance between the two objects, r = 24 cm = 0.24 m
The attractive force is given by Coulomb's law;
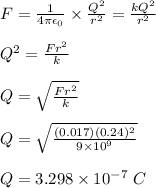
The charge of 1 electron = 1.602 x 10⁻¹⁹ C
n(1.602 x 10⁻¹⁹ C) = 3.298 x 10⁻⁷
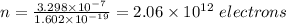
Therefore, the number of electrons transferred from one ball to the other is 2.06 x 10¹² electrons