Answer:
D. 0.75 grams
Step-by-step explanation:
The data given on the iridium 182 are;
The half life of the iridium 182,
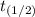 = 15 years
= 15 years
The mass of the sample of iridium, N₀ = 3 grams
The amount left, N(t) after two half lives is given as follows;
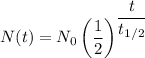
For two half lives, t = 2 ×
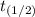
∴ t = 2 × 15 = 30
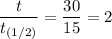
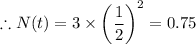
∴ The amount left, N(t) = 0.75 grams