Answer:
A mixture of 3 moles of N2, 5 moles of CO2, and 10moles of Cl2 exert a total pressure of 1120 mmHg. What is the partial pressure of CO2?
Step-by-step explanation:
According to Dalton's law of partial pressures:
The partial pressure of a gas can be determined by using the formula:
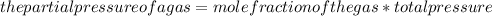
Partial pressure of CO2:
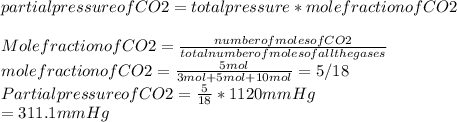
Hence, the partial pressure of CO2 is 311.1mmHg.