Answer:
D) doubles the rate
Step-by-step explanation:
The given reaction is :
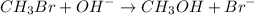
For the
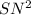 reaction, the rate of reaction depends upon the concentration of both the
reaction, the rate of reaction depends upon the concentration of both the
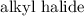 as well as the
as well as the
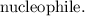
That is the rate =
![$k[CH_3Br][OH^-]$](https://img.qammunity.org/2022/formulas/chemistry/college/6siig1y8lg5d6quvce7hpnps07hnin2nsn.png)
Now if the
![$[CH_3Br]$](https://img.qammunity.org/2022/formulas/chemistry/college/xxc6bttjsj1for9294sba6tys724yr03za.png) becomes
becomes
![$2[CH_3Br]$](https://img.qammunity.org/2022/formulas/chemistry/college/vulitsuvvx5ri4v3t1zx8yg9k63mjv1gzd.png) , then the rate becomes double.
, then the rate becomes double.
So the rate' is :
=
![$2k[CH_3Br][OH^-]$](https://img.qammunity.org/2022/formulas/chemistry/college/hpbrkivim8ma35zcwt9e3l734jw62jj36v.png)
= 2 x rate
Therefore, the answer is (D) doubles the rate.