Solution :
When the temperature is held constant at 300 K, we can use the Select mass slider in order to place the weights on the lid. And we record the pressure and also the volume of the gas for each of the added mass.
Added mass on the lid Total mass Pressure Volume
0 kg 10 kg 98.1
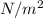
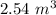
10 kg 20 kg
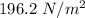
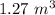
20 kg 30 kg
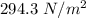
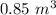
30 kg 40 kg
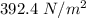
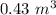
As the pressure increases at a constant temperature, the volume of the gas decreases.
Thus we can see that the pressure is inversely proportional to the volume.