Answer:
Step-by-step explanation:
Given that:
The dilution rate D = 0.28 h⁻¹
The concentration of lactose in the feed
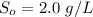
The effluent S = 0.10 g/L
Also;
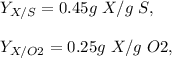
Saturation C* = 8 mg/l
To calculate the steady-state biomass, we use the formula:
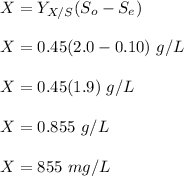
The biomass is 0.855 g/L
For a steady-state condition, the oxygen uptake rate can be illustrated by using the formula:
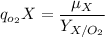
where;
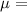 dilution rate (D)
dilution rate (D)
Thus, the steady-state can be expressed as:
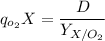
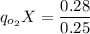
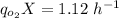
The specific rate of oxygen consumption
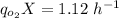
b)
In the fermentation medium, if the desired DO concentration
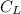 = 2 mg/L
= 2 mg/L
Here, the oxygen transfer is regarded as the rate-limiting step.
As such, the oxygen transfer rate(OTR) is equivalent to the oxygen uptake rate.
In this scenario, let's determine the oxygen transfer coefficient
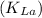 by using the formula:
by using the formula:
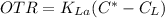
where;
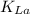 = coefficient of oxygen transfer
= coefficient of oxygen transfer
C* = saturation
Since
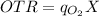
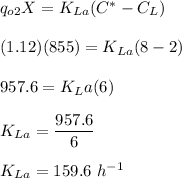
Thus, the oxygen transfer coefficient
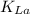 = 159.6 h⁻¹
= 159.6 h⁻¹