Answer: The statement total mass of reactants is greater than 50 g is true.
Step-by-step explanation:
According to law of conservation of mass, it is known that mass can neither be created nor it can be destroyed but it can only be transformed from one form to another.
For example,
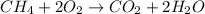
Here, mass of methane is 50 grams but oxygen is also present over here whose mass isn't given. This means that total mass of reactants is greater than 50 grams.
Thus, we can conclude that the statement total mass of reactants is greater than 50 g is true.