Answer:
-1.6 × 10⁻⁹ z
Step-by-step explanation:
To attempt this type of question, we need to first divide each charge present in the question with the smallest one.
i.e.
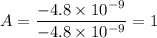
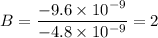
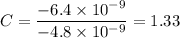
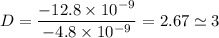
The next thing to do is to multiply each obtained value with the highest integer
A = 1 × 3 = 3
B = 2 × 3 = 6
C = 1.33 × 3 = 3.99
D = 3 × 3 = 9
Finally, we divide each charge by the result from above.
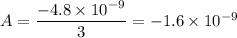
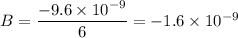
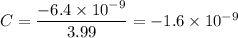
Thus, we can therefore easily conclude that the charge in zorgs (z) is:
-1.6 × 10⁻⁹ z