Answer:
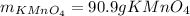
Step-by-step explanation:
Hello there!
In this case, according to the given chemical equation for the reaction for the production of potassium permanganate, we can see a 2:2 mole ratio of this product to the starting manganese (II) oxide, which means, we can calculate the theoretical yield of the former via stoichiometry:
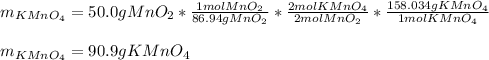
Regards!