Step-by-step explanation:
Corrections
- It should be 45 bromine atoms
- Bromine isn't found abundantly as atoms, rather as diatomic molecules
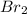
Before should show 2 phosphorus atom and 3 bromine molecules to form 2 phosphorus tribromide
The bond is covalent because both bromine and phosphorus is a non-metal