Answer:
The correct option is D.
Step-by-step explanation:
The wavelength of the photon can be calculated with the following equation:
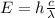
Where:
E: is the energy of the photon = 2.90 eV
h: is the Planck's constant = 6.62x10⁻³⁴ J.s
c: is the speed of light = 3x10⁸ m/s
λ: is the wavelength
Hence, the photon's wavelength is:
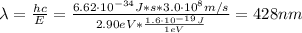
Therefore, the correct option is D.
I hope it helps you!