Answer:
2 is the numerical answer.
Step-by-step explanation:
Hello there!
In this case, according to the given information and formula, it is possible for us to remember that equation for the calculation of the average kinetic energy of a gas is:
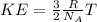
Whereas R is the universal gas constant, NA the Avogadro's number and T the temperature.
Which means that for the given ratio, we can obtain the value as follows:
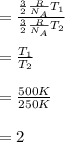
Regards!