Answer:
75.9 grams of salt
Step-by-step explanation:
The reaction is the following:
2Na(s) + Cl₂(g) → 2NaCl(s) (1)
We have:
m(Na): the mass of sodium = 30 g
V(Cl₂): the volume of the chlorine gas at STP = 60 L
So, to find the mass of NaCl we need to calculate the number of moles of Na and Cl₂.
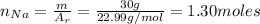
The number of moles of Cl₂ can be found by the Ideal gas law equation:
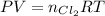
Where:
P: is the pressure = 1 atm (at STP)
R: is the gas constant = 0.082 L*atm/(K*mol)
T: is the temperature = 273 K (at STP)
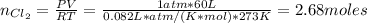
Now we need to find the limiting reactant. From the stoichiometric relation between Na and Cl₂ (equation 1), we have that 2 moles of Na react with 1 mol of Cl₂, so:
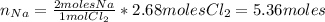
Since we have 1.30 moles of Na, the limiting reactant is Na.
Finally, we can find the number of moles of NaCl and its mass.
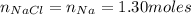
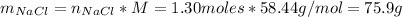
Therefore, would be formed 75.9 grams of salt.
I hope it helps you!