Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter. The formula is:
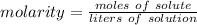
We are given grams of solute and liters of solution, so we must convert both before calculating molarity.
1. Convert Grams to Moles
We convert grams to moles using the molar mass. This value is found on the Periodic Table. It is the same as the atomic mass, but the units are grams per mole (g/mol) instead of atomic mass units (amu).
We have the compound KCl, so we look up the molar masses of the individual elements.
- Potassium (K): 39.098 g/mol
- Chlorine (Cl): 35.45 g/mol
The compound does not contain subscripts, so we can add the molar masses together to find the molar mass of the compound.
- Potassium chloride (KCl): 39.098+ 35.45= 74.548 g/mol
Use the molar mass as a ratio.
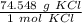
Multiply by 140 grams of KCl.
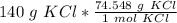
Flip the ratio so the units of grams of KCl cancel.
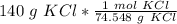
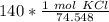
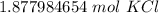
2. Convert Milliliters to Liters
1 liter contains 1000 milliliters. Create another ratio.
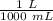
Multiply by 600 milliliters (the value we are converting).
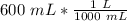
The units of milliliters cancel.

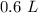
3. Calculate Molarity
Now we know the moles of solute and the liters of solution.
- 1.877984654 mol KCl and 0.6 L
Substitute the values into the molarity formula.
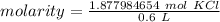
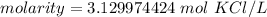
The original measurements of grams and milliliters have 2 and 1 significant figures. We must round our answer to the least number of sig figs: 1.
For the number we found, that is the ones place. The 1 in the tenths place tells us to leave the 3 in the ones place.
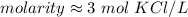
1 mole per liter is equal to 1 molar or M. Convert the units.
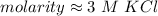
The molarity is approximately 3 M KCl.