Answer: In the reaction
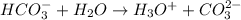 ,
,
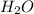 acts as a base because it has accepted a hydrogen ion or proton.
acts as a base because it has accepted a hydrogen ion or proton.
Step-by-step explanation:
According to Arrhenius, species which dissociate to give hydrogen ions when dissolved in a solvent like water are called acid.
A species which readily accepts a hydrogen ion or proton is called a base.
For example,
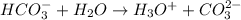
Here,
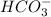 is donating hydrogen ion. So, it is an acid whereas
is donating hydrogen ion. So, it is an acid whereas
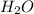 is accepting the hydrogen ion. Hence,
is accepting the hydrogen ion. Hence,
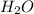 is a base.
is a base.
Thus, we can conclude that in the reaction
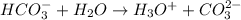 ,
,
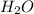 acts as a base because it has accepted a hydrogen ion or proton.
acts as a base because it has accepted a hydrogen ion or proton.