Answer:
70.137 °C
Step-by-step explanation:
The reaction generated from the question can be expressed as:
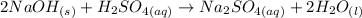
The enthalpy reaction:
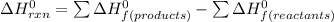
![\Delta H^0 _(rxn) =[2 * \Delta H^0 _f (H_2O) + \Delta H^0 _f (Na_2SO_4) ] -[2 \Delta * H^0 _f (NaOH) + \Delta H^0 _f (H_2SO_4) ]](https://img.qammunity.org/2022/formulas/chemistry/college/1grb81fjqoxfsabap2466ljz8j236czrw5.png)
Repacing the values of each compound at standard enthalpy conditions;
![\Delta H^0 _(rxn) =[2 * -279.4 + (-1384.49)]-[(2* -418) -913]\ kJ](https://img.qammunity.org/2022/formulas/chemistry/college/5qjfroj1dw5i5h00qrbpf2y7vi4zm825ek.png)
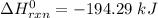
no of moles of NaOH = 12.618g/39.99 g/mol
= 0.3155 mol
no of moles of H₂SO₄ = molarity of H₂SO₄ × Volume
= 1.3553 mol/L × 100 × 10⁻³ L
= 0.13553 mol
From the reaction,
1 mol of NaOH = 2 × mol of H₂SO₄
Since mol of NaOH is greater than that of H₂SO₄, then NaOH is the excess reagent and H₂SO₄ is the limiting reactant
∴
1 mol of H₂SO₄ yields = - 194.29 kJ
0.13553 mol of H₂SO₄ will yield;
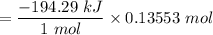
= -26.332124 kJ
= -26332.12 J
Finally,
Heat(q) =
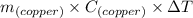
26332.12 J = 1.317 × 10³ g × 0.375 J/g°C ×ΔT
26332.12 J = 493.875 J/° C × ΔT
26332.12 / 493.875 = ΔT
ΔT = 53.317 °C
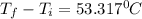
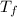 - 16.82 °C = 53.317 °C
- 16.82 °C = 53.317 °C
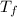 = (53.317 + 16.82) °C
= (53.317 + 16.82) °C
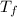 = 70.137 °C
= 70.137 °C