Answer:
__________________________________________________________
Which equation should you use?
B. P₁V₁=P₂V₂
What is the new pressure?
✔ 5000 kPa
__________________________________________________________
Step-by-step explanation:
The problem gives initial volume (2.00 L), final volume (20.0 mL), and initial pressure (50.0 kPa). Then it asks to find the final pressure. Answer B has all these variables, with P₁=initial pressure, P₂=final pressure, V₁=initial volume, and V₂=final volume. Plug the given values into the equation and then solve for the unknown value, which is P₂, final pressure.
When plugging in the values, use consistent units. 2L=2000 mL, I chose to make the consistent unit in mL. You'll get the same answer if you choose to use L as the consistent unit.
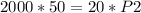
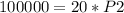
Divide both sides by 20
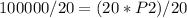
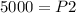
Here's a photo of Edge just incase.