Answer:
35.75 days
Step-by-step explanation:
From the given information:
For first-order kinetics, the rate law can be expressed as:
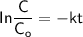
Given that:
the rate degradation constant = 0.12 / day
current concentration C = 0.05 mg/L
initial concentration C₀ = 3.65 mg/L
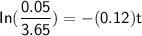
㏑(0.01369863014) = -(0.12) t
-4.29 = -(0.12)
t = -4.29/-0.12
t = 35.75 days